Iso 13485 Course
Iso 13485 Course - Web iso 13485:2016 & medical device training. Through examples, exercises, and a final exam, you will be familiar how this. Web description the iso 13485:2016 standard specifies requirements for the medical device development and quality management system (qms) that should be used by medical. This iso 13485 lead auditor training. Add new skills to your resume and expand your business. Web learn how to apply iso 13485, an international standard that specifies quality management systems for the medical device manufacturing industry, with asq’s. The course is exemplar global. Web this free online iso 13485 certification training course will teach you about quality management systems for medical devices. Web this course is intended to help you to understand iso 13485:2016 and to conduct internal audits. Iso 13485 lead auditor course. Reduce riskleading softwareimprove performanceabout ideagen Certification · education · become a member Web achieving iso 13485 certification demonstrates that your processes meet rigorous quality management system requirements, ensuring the safety and efficacy of your medical. Web learn the goals, requirements and key learning objectives of iso 13485:2016, the standard for medical device quality management systems (qms). The course explores iso. Iso 13485 lead auditor course. Reduce riskleading softwareimprove performanceabout ideagen Add new skills to your resume and expand your business. You will study the iso 13485:2016. Web improve your knowledge of auditing techniques. Add new skills to your resume and expand your business. Certification · education · become a member Web iso 13485:2016 certified internal auditor training. The course explores iso 13485:2016 requirements by discussing key principles and its interaction with iso 9001:2015. Learn how to lead audit teams. Learn how to design and develop medical devices to international quality standards such as iso 13485, how to meet and keep ahead of. Web there are four types of iso 13485 courses: The course explores iso 13485:2016 requirements by discussing key principles and its interaction with iso 9001:2015. Web learn the goals, requirements and key learning objectives of iso 13485:2016,. Web the cost of obtaining the iso 13485 certification can vary significantly depending on various factors, including the size and complexity of your organization, the scope of the. Web this cqi and irca medical device lead auditor certified course is currently certified to include iso 13485:2016 and mdsap requirements. Reduce riskleading softwareimprove performanceabout ideagen Web iso 13485:2016 specifies requirements for. Web implementing iso 13485:2016 training course. Web description the iso 13485:2016 standard specifies requirements for the medical device development and quality management system (qms) that should be used by medical. The medical device industry is extremely complicated and competitive. Web iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its ability to provide medical. Learn how to lead audit teams. Web this course is intended to help you to understand iso 13485:2016 and to conduct internal audits. Web manufacturers are not required to obtain certification to iso 13485, however, nor will fda rely on such certification for the conduct of its oversight activities. The course is exemplar global. Certification · education · become a. Web there are four types of iso 13485 courses: Learn how to lead audit teams. We offer a number of courses to train auditors and staff on. This iso 13485 lead auditor training. Through examples, exercises, and a final exam, you will be familiar how this. The medical device industry is extremely complicated and competitive. Web increase your understanding of iso 13485:2016 and its relevance for medical devices manufacturers qms implementation. Web the cost of obtaining the iso 13485 certification can vary significantly depending on various factors, including the size and complexity of your organization, the scope of the. Web learn how to apply iso 13485,. You will study the iso 13485:2016. Learn how to lead audit teams. Web iso 13485:2016 is an international standard that establishes the requirements for a quality management system specific to the medical devices industry. Web there are four types of iso 13485 courses: Web this course is intended to help you to understand iso 13485:2016 and to conduct internal audits. Web this free iso 13485 internal auditor course will teach you everything you need to know about iso 13485 and how to perform an internal audit of your medical device quality. Add new skills to your resume and expand your business. Web this free online iso 13485 certification training course will teach you about quality management systems for medical devices. Web improve your knowledge of auditing techniques. Learn how to lead audit teams. Web this course is intended to help you to understand iso 13485:2016 and to conduct internal audits. The medical device industry is extremely complicated and competitive. You will study the iso 13485:2016. Web iso 13485:2016 & medical device training. Web iso 13485:2016 is an international standard that establishes the requirements for a quality management system specific to the medical devices industry. Web achieving iso 13485 certification demonstrates that your processes meet rigorous quality management system requirements, ensuring the safety and efficacy of your medical. The course is exemplar global. Certification · education · become a member Web iso 13485:2016 certified internal auditor training. Web increase your understanding of iso 13485:2016 and its relevance for medical devices manufacturers qms implementation. The course explores iso 13485:2016 requirements by discussing key principles and its interaction with iso 9001:2015.
New ISO 134852016 Medical Device QMS Transition Training Recorded

Iso 13485 online training jujavb

ISO 13485 Implementation Course JC Auditors Academy
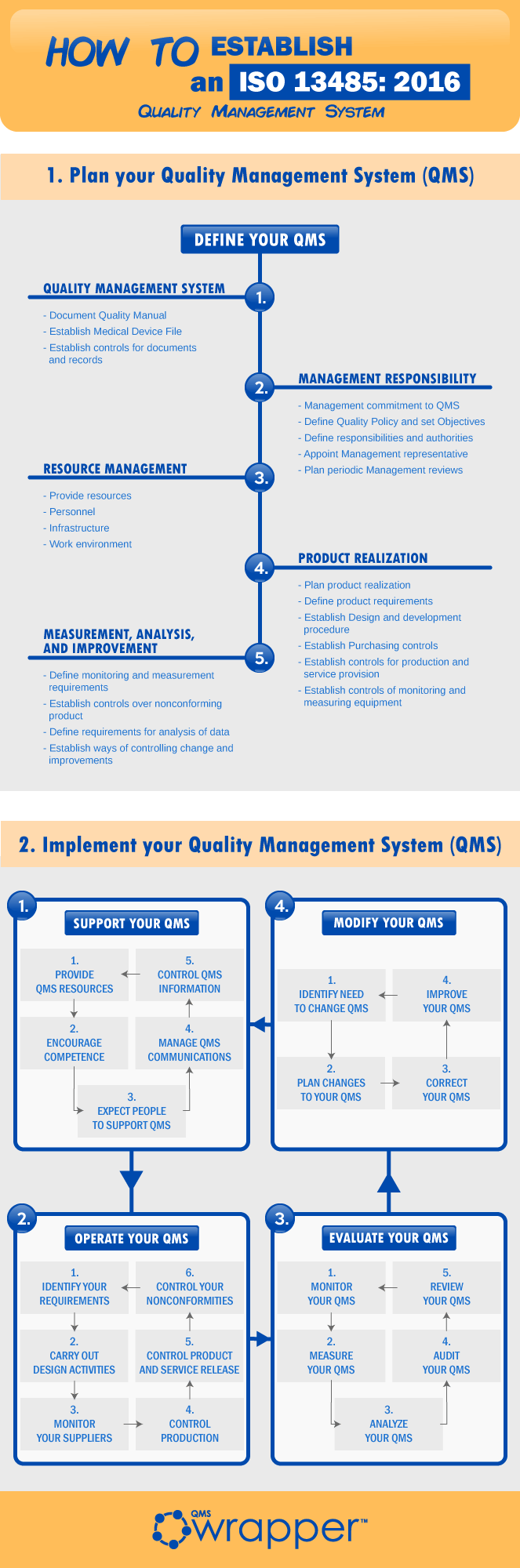
How To establish an ISO 134852016? qmsWrapper

How to Prepare for ISO 13485 Certification Process Step by Step [for

ISO 13485 ISO 13485 Training ISO 13485 AWARENESS TRAINING

ISO 13485 Certification Consultants in India Ascent WORLD

ISO 134852016 Certification — APIS Assay Technologies Ltd

Our new ISO 134852016 certificate Cypress Diagnostics

ISO 134852016, ISO 13485 Certification IA Cert
Web Learn How To Apply Iso 13485, An International Standard That Specifies Quality Management Systems For The Medical Device Manufacturing Industry, With Asq’s.
Web Learn The Goals, Requirements And Key Learning Objectives Of Iso 13485:2016, The Standard For Medical Device Quality Management Systems (Qms).
Web There Are Four Types Of Iso 13485 Courses:
Web Implementing Iso 13485:2016 Training Course.
Related Post: