Cgmp Course
Cgmp Course - Web the cgmp regulations for drugs contain minimum requirements for the methods, facilities, and controls used in manufacturing, processing, and packing of a drug product. Web cgmp course outline the certified government meeting professional designation (cgmp) is designed for planners and suppliers whose work is governed by the rules and regulations of the federal government. Module 5 • 3 hours to complete. Web $23,904 usd tuition total tuition for other mba programs can easily exceed $200,000. With the imba, you'll save substantially on your total cost while pursuing an mba from a leading program. Web the certified pharmaceutical gmp professional understands the good manufacturing practices (gmp) as regulated and guided by national and international agencies for the pharmaceutical industry. Whether it is solo learning or group learning, we have many years of experience in managing training projects of any size. Providing training on validation processes and equipment qualification. This course provides an intensive conceptual and applied introduction to auditing in society. Web the certified government meeting professional designation (cgmp) is designed for planners and suppliers whose work is governed by the rules and regulations of the federal government. Web cgmp compliance:popular programs & training categories. Web there are 5 modules in this course. Prepare for the gmpro™ certification at biopharma institute today! Current good manufacturing practice (cgmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. Web this course provides an introduction to gmp for pharmaceuticals and the current u.s. Individuals who have earned their cgmp have obtained the highest designation available that is specifically for government meeting. Module 5 • 3 hours to complete. Web the gmp academy is offering various online trainings in the coming months: This course provides an intensive conceptual and applied introduction to auditing in society. The registration fee for each course. Module 5 • 3 hours to complete. This course provides an intensive conceptual and applied introduction to auditing in society. Web cgmp compliance:popular programs & training categories. Web we cater to training projects of any size: It covers key principles of the who and pic/s standards and will equip participants with a broad, foundational understanding of gmp. In this module, we will learn about advanced concepts in supply chain management. Web this course provides an introduction to gmp for pharmaceuticals and the current u.s. Cgmp training course is designed for anyone who needs a good understanding of current good manufacturing practices. Web the gmp academy is offering various online trainings in the coming months: It is designed. Web validation and qualification training: Web the certified government meeting professional designation (cgmp) is designed for planners and suppliers whose work is governed by the rules and regulations of the federal government. The registration fee for each course. Introduction to good manufacturing practice (gmp) course includes sections on: It is designed to minimize the risks involved in any pharmaceutical production. If a scheduling conflict arises, you are free to take a term off and won’t be charged during that term. This accredited online training course will teach you about good manufacturing practice (cgmp) which is a system for ensuring that products are consistently produced and controlled according to quality standards. It is designed to minimize the risks involved in any. It is designed to minimize the risks involved in any production that cannot be eliminated through testing the final product. Web the certified government meeting professional designation (cgmp) is designed for planners and suppliers whose work is governed by the rules and regulations of the federal government. Individuals who have earned their cgmp have obtained the highest designation available that. It is designed to minimize the risks involved in any production that cannot be eliminated through testing the final product. Web the full length course includes an introduction to current good manufacturing practices (cgmps), information on maintaining product quality, the scope of gmp rules, a section on cleaning and sanitation, a look into proper documentation, and concludes with a. If. Web this course provides an introduction to gmp for pharmaceuticals and the current u.s. Using web based training, the course provides a breakdown of the current good manufacturing practice requirements from. It is designed to minimize the risks involved in any pharmaceutical production that cannot be eliminated through testing. Introduction to current good manufacturing practices (cgmps) maintaining product quality scope. Web the gmp academy is offering various online trainings in the coming months: It covers key principles of the who and pic/s standards and will equip participants with a broad, foundational understanding of gmp. Cgmp professional certification (pharmaceuticals) gmp: Each year, estimates suggest that 1/3 of all food produced is lost or wasted, making postharvest loss. Introduction to current good. Whether it is solo learning or group learning, we have many years of experience in managing training projects of any size. Individuals who have earned their cgmp have obtained the highest designation available that is specifically for government meeting professionals. Teaching employees how to identify, assess, and mitigate risks. This course provides an intensive conceptual and applied introduction to auditing in society. To view our complete cgmp training course catalog, click here. Web the certified pharmaceutical gmp professional understands the good manufacturing practices (gmp) as regulated and guided by national and international agencies for the pharmaceutical industry. Web there are 5 modules in this course. Cgmp provides for systems that assure proper design, monitoring, and control of manufacturing processes. Explaining risk assessment and management processes within cgmp. With the imba, you'll save substantially on your total cost while pursuing an mba from a leading program. Web the certified government meeting professional designation (cgmp) is designed for planners and suppliers whose work is governed by the rules and regulations of the federal government. Web cgmp refers to the current good manufacturing practice regulations enforced by the fda. Learn more, view the details for cgmp online. Each year, estimates suggest that 1/3 of all food produced is lost or wasted, making postharvest loss. (2 or 4 credit hours) Using web based training, the course provides a breakdown of the current good manufacturing practice requirements from.
Advanced cGMP Training For Leaders & Top Management The Health, Drug

GMP Olympics cGMP Training The Health, Drug, Prescription, and GMP

Qualstar Simulation An Advanced CGMP Training Course The Health

PPT FDA cGMP Training Program PowerPoint Presentation, free download

cGMP Training, Current Good Manufacturing Practices, FDA cGMP, QSR and

Introduction to Good Manufacturing Practices (cGMP) Training
Introduction To CGMP Compliance Course PDF Tablet (Pharmacy
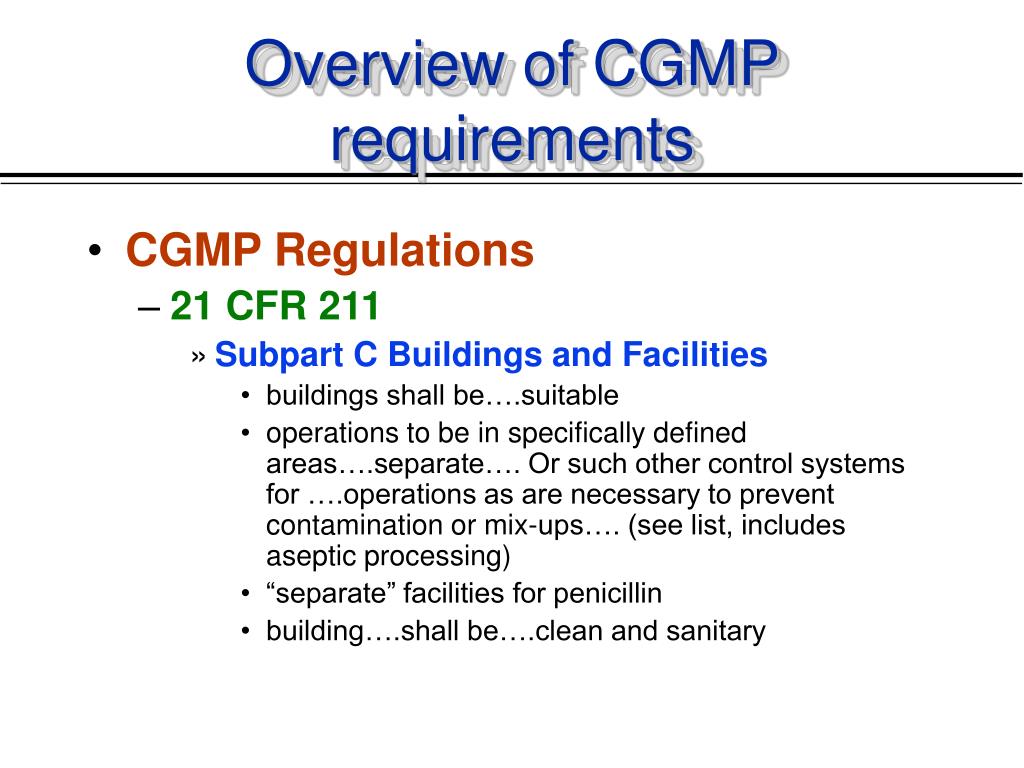
PPT FDA cGMP Training Program PowerPoint Presentation, free download

cGMP Training 2013

PPT FDA cGMP Training Program PowerPoint Presentation, free download
This Course Provides An Overview Of The Issue Of Postharvest Loss Of Grains By Exploring Essential Physical, Technical, And Social Dimensions Of Postharvest Supply Chains And Loss Prevention Methods Globally.
It Reviews A Brief History Of Gmp Regulations And Discusses The Regulatory Requirements For The Quality Management System, Equipment, Batch Records, Validation, Packaging, Labeling, Holding, Distribution, And Audits.
Cgmp Covers All Aspects Of Production From.
Web Each Cgmp Certification Training Course Is Designed To Present And Explain Cgmp Mandates, As Well As To Provide Comprehensive Analysis And Instruction On How To Best Comply.
Related Post:
